The article was first published on the Kluwer Trademark Blog
While the new EUTMR 2017/1001 deleted any reference to disclaimers – previously provided by Article 37(2) of EUTMR No. 207/2009 – both the EU Directive 2008/95 and the Recast Directive 2015/2436 neither allowed nor prohibited disclaimers at national level. Few Member States had disclaimers on their book (Sweden, Ireland and Latvia) and from Sweden the question raised was “what role may disclaimers play in the assessment of likelihood of confusion?”. On 12 June 2019, the CJEU basically answered this with “none” (Case C-705/17).
The Swedish mark “ROSLAGS PUNSCH”
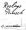
for alcoholic drinks in class 33 was granted in 2007 by the Swedish Trademark Office (PRV), with a disclaimer over the word “RoslagsPunsch”, because “Roslags” is a geographical indication in Sweden.
In 2015, Mats Hansson filed a trademark application for “ROSLAGSÖL” for non-alcoholic beverages and beers in Class 32, but the PRV rejected it because of likelihood of confusion between this sign and the earlier trademark “ROSLAGS PUNSCH”. [Note that öl is beer in Swedish.]. The PRV justified its refusal saying to have changed its practice on geographical names following the Windsurfing Chiemsee judgment (C‑108/97 and C‑109/97), so that geographical names would only be considered descriptive when designating a place associable with the category of goods concerned. Accordingly, for the PRV, “ROSLAGS” is now considered to be a fanciful term for alcoholic beverages, and thus ROSLAGSÖL” could cause confusion among the public with the earlier mark “ROSLAGS PUNSCH”.
Mr. Hansson appealed to the Patents and Market Court, arguing that “ROSLAGS” was descriptive of a geographical region and it was in the public interest that it remains available to traders. The Patents and Market Court reversed the PRV’s ruling and admitted ROSLAGSÖL to registration. The PRV thus appealed before the Svea Court of Appeal, (Stockholm), which decided to stay the proceedings to ask the CJEU if Art 4(1)(b) of the EU Directive precludes national provisions to permit disclaimers whose effect would be to exclude an element of a complex trademark from the assessment of likelihood of confusion or to attribute to such an element, in advance and permanently, limited importance in that analysis.
The CJEU held that disclaimers, although theoretically admissible, cannot affect the objectives of the EU directive, that is to ensure the same conditions for registration and equal protection of trademarks in the EU. Accordingly, a disclaimer that excludes a descriptive or non-distinctive element of a trademark from the analysis of the relevant factors for assessing the likelihood of confusion is not compatible with the EU system, as it would cause an incorrect assessment concerning both the similarity between the signs and the distinctiveness of the earlier trademark.
The principle of interdependence between the relevant factors is directed to reflect as much as possible the actual perception of the public. In light of this intent, the functioning of a trademark as an indication of origin must be assessed taking into consideration all its components regardless their distinctiveness.
The CJEU also observed that the exclusion of an element from the analysis of the distinctiveness of a trademark may modify its scope of protection and it could lead to registration of signs liable to cause likelihood of confusion (also because most EU Member States do not allow disclaimers). Moreover, non-distinctive or weak elements have already less impact in the analysis of the similarity between the signs. Finally, EU law provides enough guarantees to ensure that descriptive signs are not registered and may thus be freely used by other economic operators and the exclusive rights conferred by a mark do not allow its proprietor to prohibit third parties from using in the course of trade descriptive indications.
Therefore, the CJEU held national legislation contrary to EU law that provided for disclaimers, the effect of which would be to exclude an element of a complex trade mark from the global analysis of a likelihood of confusion or to attribute to such an element, in advance and permanently, limited importance in that analysis.
While after this decision we can effectively kiss good bye to disclaimers, the CJEU’s arguments on the “limited value” of descriptive or weak elements does not always appear to find confirmation in practice. EU case law, especially, but not exclusively, in the pharma sector (see for instance Sebotherm/SeboCalm – T-441/16, ImmunoStim/Immunostad – T-403/16 and Mundipharma/Multipharma – T-144/16), shows instead a good number of cases where courts found likelihood of confusion, although the similarity only derived from weak or descriptive elements. With that case law in mind, perhaps disclaimers were not such a bad idea after all ….
July 2019